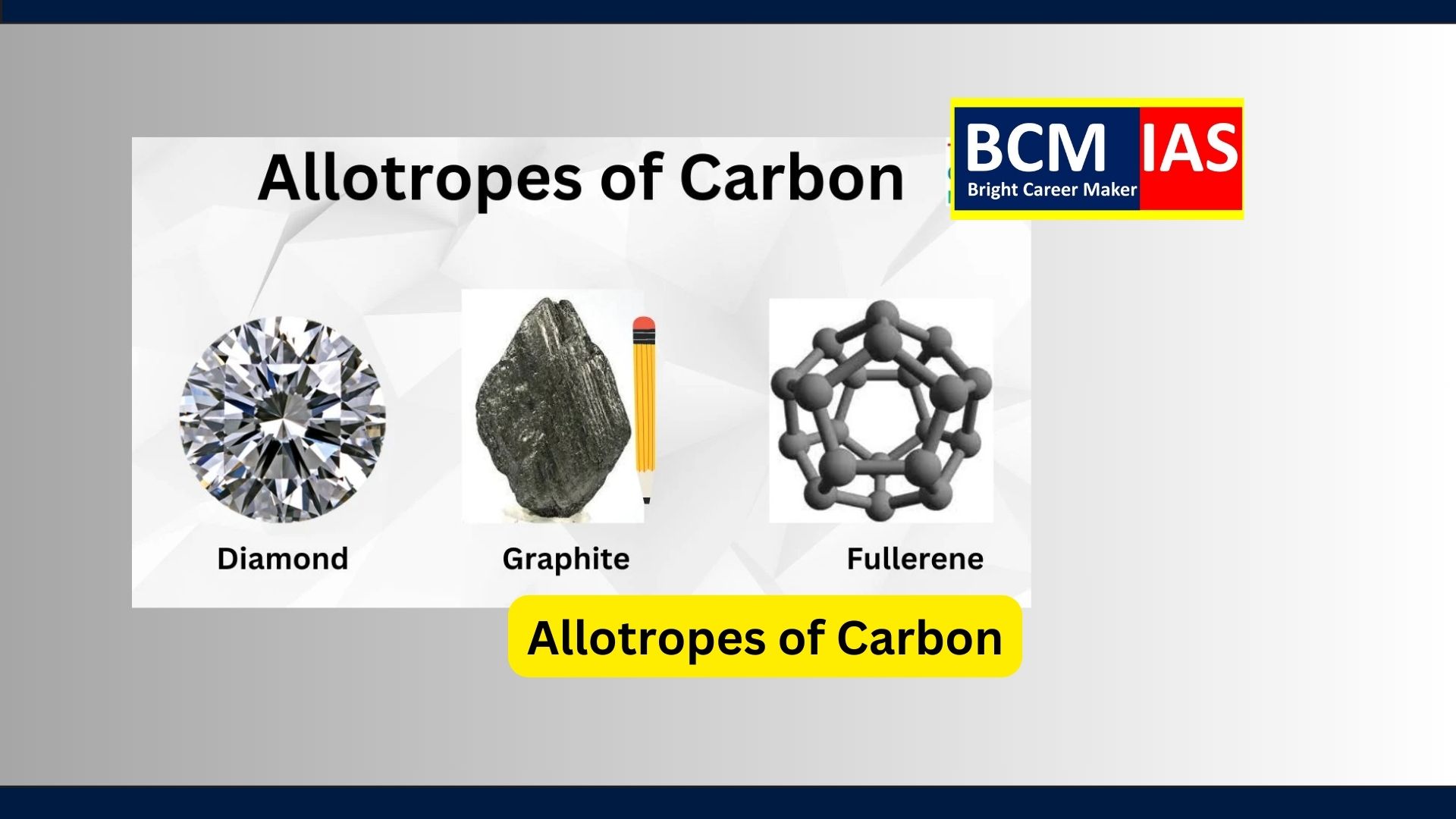
Allotropes of Carbon
Context
Carbon, a fundamental element in
chemistry and materials science, exhibits multiple allotropes with diverse
properties. These allotropes, such as diamond, graphite, graphene, and
fullerenes, play pivotal roles in industrial, scientific, and technological
advancements.
What are Allotropes of Carbon?
- Definition: Allotropes are different
forms of the same element in the same physical state with varying atomic
arrangements and properties.
- Primary Allotropes:
Diamond, Graphite, Fullerenes, and Graphene.
- Other Forms: Carbon nanotubes and
amorphous carbon (e.g., charcoal, soot).
Allotropes of Carbon and Their
Properties
1. Graphite:
o Structure:
Two-dimensional sheets of hexagonal carbon layers; each carbon atom is bonded
to three others.
o Properties:
§ Electrical
Conductivity: Good conductor due to delocalized electrons.
§ Softness: The
softest carbon allotrope; layers slide over one another, acting as a lubricant.
o Uses:
§ Solid
lubricant in machinery.
§ Electrodes
in batteries and other electrical applications.
2. Graphene:
o Structure:
Single-layer sheet of carbon atoms from graphite.
o Properties:
§ Strong,
lightweight, and electrically conductive.
§ High
surface area and biocompatibility.
o Uses:
§ Electronics,
energy storage, biomedical devices, and sensors.
3. Diamond:
o Structure:
Three-dimensional tetrahedral arrangement of carbon atoms.
o Properties:
§ Hardness: Hardest
naturally occurring material; suitable for industrial cutting and drilling.
§ Thermal
Conductivity: Excellent heat sink but lacks electrical conductivity.
§ Transparency: Valued
for jewelry.
o Lab-grown
Diamonds (LGDs):
§ Artificially
created using graphite as a seed.
§ Identical
to natural diamonds in physical properties.
4. Fullerene:
o Structure: Cage-like
molecular structure resembling a football (e.g., Buckminsterfullerene C60).
o Properties:
§ High
electrical and thermal conductivity.
o Uses:
§ Semiconductors,
superconductors, lubricants, and reinforcing materials.
5. Carbon
Nanotubes:
o Structure:
Cylindrical rolled-up graphene sheets.
o Properties:
§ Lightweight,
strong, and electrically conductive.
§ Biodegradable.
o Uses:
§ Electronics,
nanotechnology, drug delivery, and water purification.
6. Amorphous
Carbon:
o Structure:
Non-crystalline forms like charcoal and soot.
o Uses:
§ Activated
carbon for filtration and adsorption.
Importance of Allotropes
1. Industrial
Applications:
o Diamonds
for cutting, grinding, and heat management.
o Graphite
and graphene in electronics and batteries.
2. Biomedical
Innovations:
o Graphene
and nanotubes for drug delivery and tissue engineering.
3. Environmental
Benefits:
o Activated
carbon for water purification.
o Carbon
nanotubes for renewable energy storage.
PYQs:
o 2020
Question on Carbon Nanotubes: Highlighted their biomedical and
environmental roles.
o 2012
Question on Graphene: Emphasized its electrical and mechanical properties.
Conclusion
The allotropes of carbon, ranging
from diamonds to graphene, exemplify the versatility of this element. Their
unique properties and applications in diverse fields make them critical for
scientific innovation, industrial use, and sustainable technologies.
Understanding their significance is essential for both scientific and
competitive exam preparation.
Mains Question:
Discuss the
significance of the different allotropes of carbon in industrial, scientific,
and environmental applications. How do their unique properties contribute to
their diverse uses?
Answer:
Introduction
Carbon is a versatile element with
various allotropes, each possessing distinct atomic arrangements and
properties. These allotropes, such as diamond, graphite, graphene, fullerenes,
and carbon nanotubes, have revolutionized fields ranging from industrial
manufacturing to biomedical engineering and environmental sustainability.
Body
Significance of Allotropes of
Carbon
1. Industrial
Applications:
o Diamond:
§ Hardness: The
hardest natural material; used in cutting, drilling, and grinding tools.
§ Thermal
Conductivity: Effective in heat dissipation in electronic devices.
o Graphite:
§ Conductivity: Used in
electrodes, batteries, and electric arc furnaces.
§ Lubrication: Solid
lubricant in machinery.
2. Scientific
Innovations:
o Graphene:
§ Strength
and Conductivity: Ideal for next-generation electronics, flexible
displays, and quantum computing.
§ Biocompatibility: Used in
drug delivery and tissue engineering.
o Carbon
Nanotubes:
§ Nanotechnology:
Revolutionizing fields like electronics, sensors, and energy storage.
§ Biomedical
Applications: Drug delivery systems and artificial blood capillaries.
3. Environmental
Applications:
o Amorphous
Carbon:
§ Activated
Carbon: Used in water purification and air filtration systems.
o Carbon
Nanotubes:
§ Renewable
Energy: Enhances efficiency in solar cells and batteries.
§ Biodegradability:
Sustainable alternative in various technologies.
4. Other Uses:
o Fullerenes:
§ Superconductors: Critical
in advanced electrical systems.
§ Catalysts:
Facilitate chemical reactions in industrial processes.
Unique Properties Contributing to
Diverse Uses
1. Diamond:
o Strong
covalent bonds confer hardness.
o Transparent
nature makes it valuable for jewelry.
2. Graphite:
o Layered
structure allows easy sliding, ideal for lubrication.
o Delocalized
electrons enable electrical conductivity.
3. Graphene:
o Single-atom
thickness ensures high surface area and flexibility.
o Exceptional
mechanical strength and conductivity.
4. Carbon
Nanotubes:
o Cylindrical
shape and nanoscale dimensions provide high strength-to-weight ratio.
o Ability to
conduct electricity and heat efficiently.
Conclusion
The allotropes of carbon exemplify
the adaptability of this element to meet diverse technological and industrial
demands. From diamonds in cutting tools to graphene in futuristic electronics,
their applications underline the importance of understanding material
properties for innovation. Harnessing these allotropes responsibly can drive
sustainable development while addressing scientific and environmental
challenges.
Value
Addition
- Quote:
"Carbon is not just the element of life but also the element of
innovation."
- Fact:
Graphene is 200 times stronger than steel and conducts electricity better
than copper.
MCQs
1. With reference to carbon
allotropes, consider the following statements:
1. Graphite is
a good conductor of electricity due to the presence of free electrons.
2. Diamond
conducts electricity because of its strong covalent bonds.
3. Graphene is
a single layer of carbon atoms arranged in a hexagonal lattice.
Which of the statements given above
is/are correct?
(a) 1 and 2 only
(b) 1 and 3 only
(c) 2 and 3 only
(d) 1, 2, and 3
Answer: (b)
2. Which of the following are
properties of carbon nanotubes? (Adapted from UPSC Prelims 2020)
1. They are
used in drug delivery systems.
2. They have
high electrical conductivity.
3. They are
biodegradable in nature.
Select the correct answer using the
code given below:
(a) 1 and 2 only
(b) 1 and 3 only
(c) 2 and 3 only
(d) 1, 2, and 3
Answer: (d)
3. Graphene is frequently in news.
What makes it significant? (Adapted from UPSC Prelims 2012)
1. It is one
of the strongest materials tested so far.
2. It has high
optical transparency.
3. It is
entirely made of silicon.
4. It can be
used as conducting electrodes for touch screens.
Which of the statements given above
are correct?
(a) 1 and 2 only
(b) 1, 2, and 4 only
(c) 3 and 4 only
(d) 1, 2, 3, and 4
Answer: (b)
4. With reference to fullerenes,
consider the following statements:
1. Buckminsterfullerene
(C60) has a cage-like structure resembling a football.
2. Fullerenes
are used in the development of superconductors and catalysts.
3. They are
naturally occurring and cannot be synthesized artificially.
Which of the statements given above
is/are correct?
(a) 1 and 2 only
(b) 1 and 3 only
(c) 2 and 3 only
(d) 1, 2, and 3
Answer: (a)
5. Which of the following is NOT an
application of activated carbon?
(a) Water purification
(b) Air filtration
(c) Heat dissipation in electronics
(d) Removal of impurities in chemical processes
Answer: (c)



Comments on “Allotropes of Carbon”